Synthesis and in vitro antimicrobial, antiprotozoal and antitumor evaluation of phenothiazine linked 1,2,4-triazoles
Keywords:
1,2,4-triazole, phenothiazine, antibacterial, antifungal, antiprotozoal, antitumorAbstract
Aim: The purpose of this study is to create compounds containing phenothiazine triazoles and test them against various bacterial, fungal, protozoan, and M. tuberculosis H37Rv species. Anti-tumor efficacy is also included.
Background: Phenothiazine comprises two separate hetero atoms, Sulphur and nitrogen, and the 1,2,4-triazoles nucleus has been identified as an antifungal agent with three nitrogen-containing heterocyclic rings. In medicinal chemistry, the nucleus is significant on both counts. Both of these heterocycles are associated in this study with enhancing the therapeutic value.
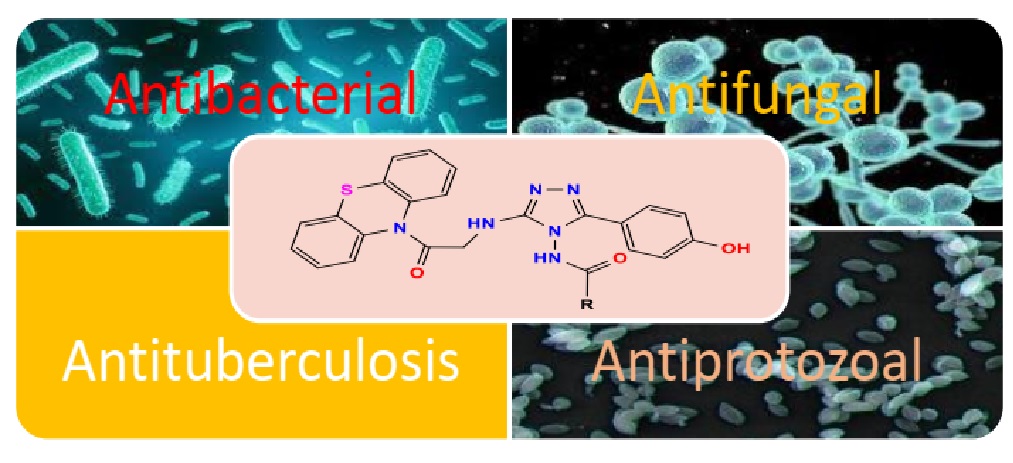
Objective: Phenothiazine is widely known for its antibacterial, antihistamine, sedative, spasmolytic, and anticancer activities. 1,2,4-triazoles nucleus is the active component of antifungal medications. When these two heterocycles are joined, the biological profile may be improved. The primary goal of this study is to assess the various biological activities of freshly synthesized 1,2,4-triazoles including phenothiazine.
Method: Condensation of 2-chloro-5-(4-hydroxyphenyl)-1-(phenothiazin-10-yl)-ethanone-1 is the method. -2-amino-1,3,4-oxadiazole-2 is then used to create a range of phenothiazine-linked triazoles (5a–m) by reacting with different acid hydrazides. All recently synthesized substances have their structural integrity confirmed using data from IR, 1H-NMR, 13C-NMR, and mass spectrometry. All synthesized substances were evaluated for their antimicrobial, antiprotozoal, and anticancer activities. By connecting the nitrogen of phenothiazine to monocyclic heterocycles like oxadiazole and triazole via the alkyl chain showed significant biological activity of the newly synthesized compounds 5a–m.
Result: Biochemical testing revealed that compounds 1, 2, 3, 5a, 5d, 5e, 5f, 5k, and 5l had antibacterial activity. The antifungal activity of vitamins 5b, 5c, 5d, 5f, and 5k is active. Active against M. tuberculosis are 5d and 5k. In comparison to normal medications, 5a, 5i, 5j, 5k, and 5l showed higher percentages of lysis on T. Cruzi. The anti-tumor effects of 5a, 5j, and 5l were present.
Conclusion: Antibacterial, antifungal, antiprotozoal, and antitumor activities have all been assessed in this study. The majority of recently created compounds have been shown to be biologically active agents. The primary emphasis of this research is the novel compounds' anti-protozoal action
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Chemical Synthesis Letters

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.




